Artificial Intelligence–Guided Ultrasound Intervention Device
When a person is experiencing a traumatic injury, interventions to avoid fatal hemorrhaging must be applied on site until the person can be evacuated to a hospital. The insertion of a catheter into a central blood vessel, through which fluids, drugs, or other aids can be introduced, is a life-saving measure to manage hemorrhaging. However, medics or EMTs, often the first to provide medical assistance in emergency settings, are not trained to do this procedure, which requires precision and control to gain access to a deep, major vessel. The ability to provide this aid on site could be transformative: hemorrhage is the leading cause of preventable death for both military and civilian trauma.
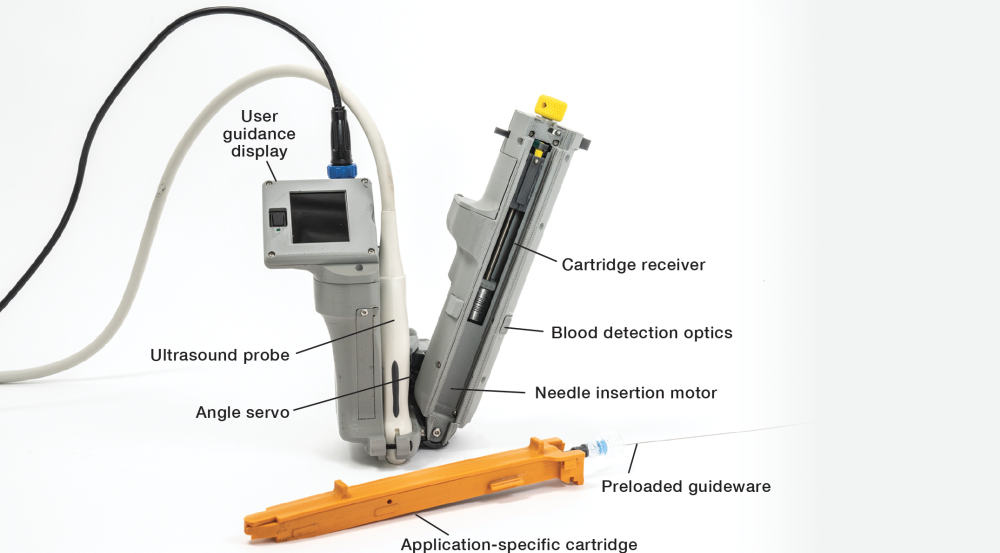
To help medics save lives under these circumstances, Laboratory researchers and physicians at Mass General Brigham developed AI-GUIDE. Using state-of-the-art artificial intelligence (AI) and robotics, this handheld catheterization tool guides users to perform the key step of inserting a needle and guidewire in the targeted vessel, after which they can readily complete catheterization.
To use the system, the medic/EMT plugs a commercial portable ultrasound probe into the AI-GUIDE tool. They then place the ultrasound probe on the patient's body near the targeted vessel (AI-GUIDE is currently optimized to locate the femoral vessels, near the upper thigh). As the user moves the probe, the Laboratory's AI-powered software uses the ultrasound imagery to detect the vessel in real time. A screen on the tool displays a simple dot-and-crosshairs guide that shows when the tool's needle is properly positioned above the vessel. After the software conducts a final safety check, the user is instructed to push the needle-insertion button. The tool automatically confirms successful insertion by detecting blood flowing to the syringe, at which point the medic/EMT inserts a preloaded guidewire followed by a catheter.
AI-GUIDE's vessel detection and tracking, and reliable needle and guidewire insertion, have been demonstrated on phantoms (physical models of tissue and blood vessels) and pigs. This technology is now ready to transition to industry; once transitioned, its licensee can seek Food and Drug Administration approval.




